[ad_1]
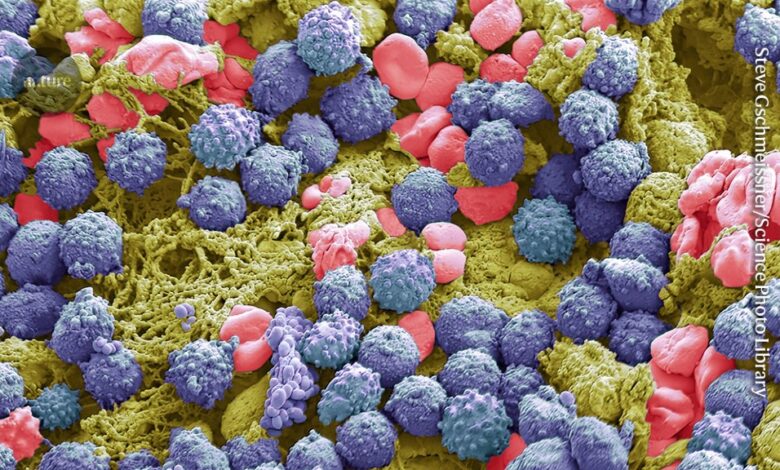
Immune cells (purple, artificially coloured) can contribute to chronic inflammation.Credit: Steve Gschmeissner/SPL
The latest generation of anti-obesity drugs has taken the world by storm, thanks to their effectiveness at treating diabetes and reducing weight. But these drugs also have a less well-known superpower: the ability to suppress inflammation.
Evidence suggest that the drugs classified as GLP-1 receptor agonists — a category that includes brand names such as Mounjaro and Wegovy — can reduce inflammation in the liver, kidneys and heart. The drugs even seem to dial down inflammation in the brain, leading scientists to hope that the compounds could be used to treat Parkinson’s and Alzheimer’s diseases, both of which are characterized by brain inflammation. A recent review1 listed more than 20 clinical trials that are exploring the drugs as therapies for the two conditions.
“The next generation of drugs could be even more targeted to reduce these new inflammation pathways that we’ve identified,” says Daniel Drucker, an endocrinologist at the University of Toronto in Canada who co-authored a study2 investigating how the drugs dampen inflammation that was published last month. “Maybe they would be more effective.”
Body-wide effects
The GLP-1 receptor agonists include semaglutide, which is marketed as Wegovy for obesity and Ozempic for diabetes, and tirzepatide, marketed as Mounjaro for diabetes and Zepbound for obesity. The drugs mimic a gut hormone called glucagon-like peptide 1 (GLP-1), which acts on the brain to dampen appetite, in addition to controlling blood sugar levels. But a slew of findings, many made in the past few years, showcase the ability of the hormone and its mimics to calm inflammation, caused by an onslaught of immune cells and immune-system chemicals.
Anti-obesity drugs’ side effects: what we know so far
In one experiment, a GLP-1 receptor agonist called liraglutide alleviated liver inflammation in mice with a fatty liver3. A similar effect was observed in a pilot study in people4. In other experiments in mice, liraglutide demonstrated anti-inflammatory potential in the kidneys5 and the heart6. And GLP-1 itself reduces inflammation in fat tissue in obese and diabetic mice7.
“We know from animal studies and human studies that GLP-1 seems to reduce inflammation almost everywhere,” says Drucker.
The reductions in body weight and blood sugar that the drugs trigger probably help to control inflammation. But some of the drugs’ anti-inflammatory effects start even before meaningful weight loss is achieved. This is why scientists think there’s a separate mechanism at play.
Brain power
Drucker and his colleagues noticed a potential clue: receptors for GLP-1 are scarce in immune cells in many tissues in which the hormone and its mimics reduce inflammation, but are abundant in the brain. To test the nervous system’s role, Drucker’s team began by inducing system-wide inflammation in mice.
Why BMI is flawed — and how to redefine obesity
“Multiple GLP-1 drugs made those mice better and reduced inflammation,” Drucker says. But when the researchers used either genetic methods or drugs to block GLP-1 receptors in the animals’ brains, the GLP-1 drugs no longer reduced inflammation in multiple tissues. The findings were published in Cell Metabolism in December2.
The paper helps to move the field forwards by demonstrating that, at least in mice, the drugs’ anti-inflammatory effects are achieved directly through GLP-1 receptors and are mediated by the brain, says Nigel Greig, a pharmacologist at the National Institutes of Health in Baltimore, Maryland. He notes that previous studies8 have established that only a small amount of these drugs can actually enter the brain. “It’s quite remarkable that the brain entry is so low, but it’s so important for anti-inflammatory action, both systemically and within the brain,” Greig says.
Targeting pathological proteins
The GLP-1 drugs’ anti-inflammatory powers have promise for treating neurodegenerative diseases such as Parkinson’s and Alzheimer’s. Both are characterized by neuroinflammation that is not effectively targeted by current therapies. And in both disorders, pathological proteins — for example, beta-amyloid in Alzheimer’s and alpha-synuclein in Parkinson’s — interact with certain receptors in the brain to induce a cascade of events that cause inflammation.
Excessive inflammation can contribute to disease, Greig says. But GLP-1 receptor agonists seem to have the ability to knock back inflammation in the brain so that important processes, such as the birth of new neurons, can continue to occur, he notes.
Four key questions on the new wave of anti-obesity drugs
In one clinical trial, a GLP-1 receptor agonist called exenatide led to greater improvement in the motor abilities of people with Parkinson’s than did a placebo8. A trial is now assessing the medication in a larger population of people with Parkinson’s, and should conclude this year. Meanwhile, at least two clinical trials are testing semaglutide as a therapy for early-stage Alzheimer’s disease.
The drugs’ anti-inflammatory action might also help to boost their effectiveness against diabetes and obesity, says Vinicius de Frias Carvalho, a biologist at the Inflammation Laboratory at the Oswaldo Cruz Institute in Rio de Janeiro, Brazil. Both conditions “are also inflammatory diseases”, he says. Semaglutide’s anti-inflammatory action might play a part in an effect that recently made headlines: the drug provides strong protection against cardiovascular disease in people with obesity.
The use of GLP-1 drugs to treat inflammation-related diseases could expand even further, Greig says, especially given the drugs’ lack of significant side effects. “There are so many systemic disorders where there’s an inflammatory component,” he says. It only makes sense, he says, to try the drugs against such disorders if there’s no effective treatment.
Source link